CASE
The world’s first aseptic powder handling system
Founded in 1968, SKAN is a pioneer in the field of clean room equipment and isolator design for the global pharmaceutical industry. Innovative products, customer-specific solutions, and a powerful service organization have made SKAN a market leader and an important partner for industry and research laboratories (Source: www.skan.ch). Together with SKAN, Robotec Solutions developed a world first in aseptic production for one of the largest manufacturers of pharmaceutical products.
-
Task
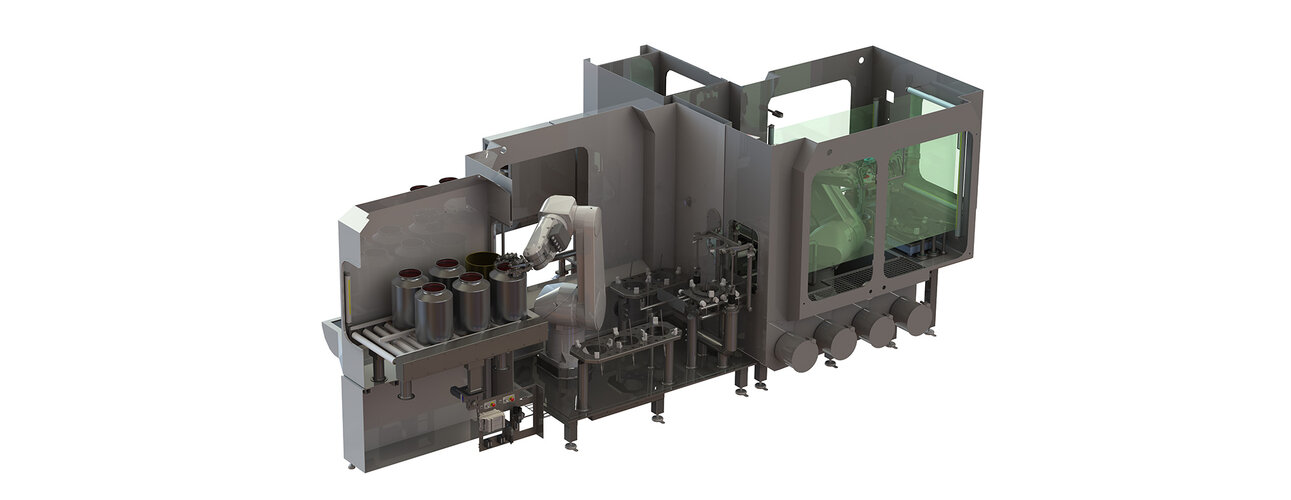
The system needs to empty a batch of five cannisters of powder into a mill while maintaining sterility within the isolator. Moreover, product and process security are also exceedingly important.
-
Solution
The cannisters are transported via roller conveyor into the SARA material lock, where they are decontaminated for 30 minutes. After decontamination, the first robot removes the cannisters from the SARA und places them in the buffer positions in the first isolator. In order for the cannisters to be emptied in the second isolator, the robot removes the lid from the cannister like a can opener. It then pushes the cannister onto a trolley in the second isolator.
The plug is removed from the cannister in the second isolator – the product is now open. The second robot grips the cannister, presents it to a barcode scanner, and then weighs it. Now the cannister is emptied into a mill – this process is carried out by a butterfly valve that is integrated into the gripper. Once it has been fully emptied, the cannister is transported out of the system through a final airlock.
<iframe width="560" height="315" src="https://www.youtube.com/embed/13ybl9IdO6M?controls=0" frameborder="0" allow="accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture" allowfullscreen></iframe>
-
Conclusion
Thanks to smart engineering and the targeted use of robot technology, we were able to implement a powerful system that complies with GMP and FDA guidelines in an extremely compact space
-
Special features
- Documentation and validation in accordance with GMP
- Entire system can be decontaminated with hydrogen peroxide (H2O2)
- Easy to clean thanks to the use of top-quality materials paired with smart engineering
- A high degree of process reliability thanks to the use of robot technology

Jörg Lanz, Sales